Three Key Considerations for Specialty Pharmacy Benefits Management: Price, Clinical Appropriateness, and Drug Mix
Managing specialty pharmacy benefits has become a critical challenge for self-insured employers and benefit consultants alike. With specialty drugs accounting for over 50% of pharmacy spend, it’s essential to balance cost management with patient outcomes. Here are three key considerations for specialty pharmacy benefits management: price, clinical appropriateness, and drug mix.
Price: Breaking Down the True Cost
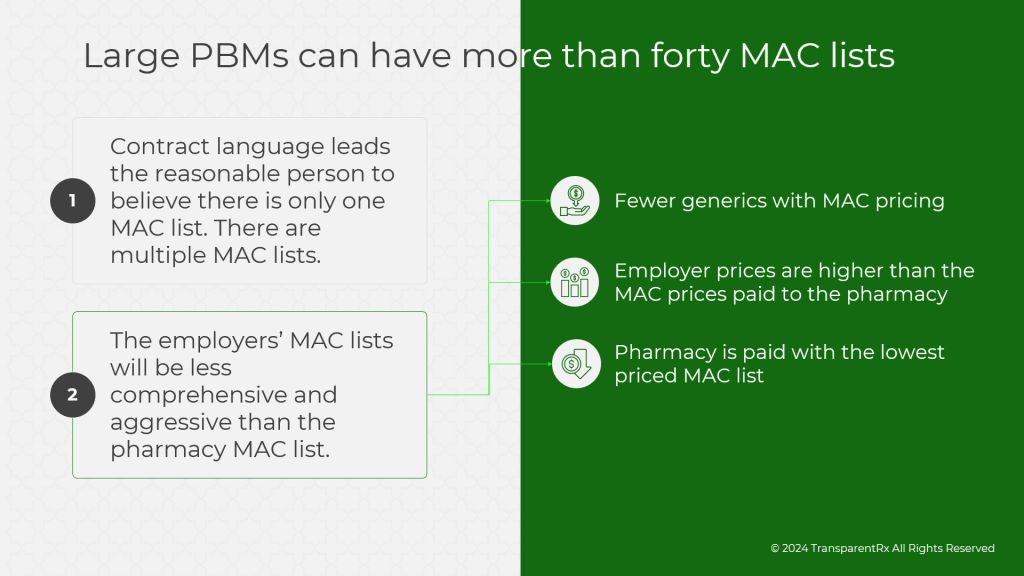
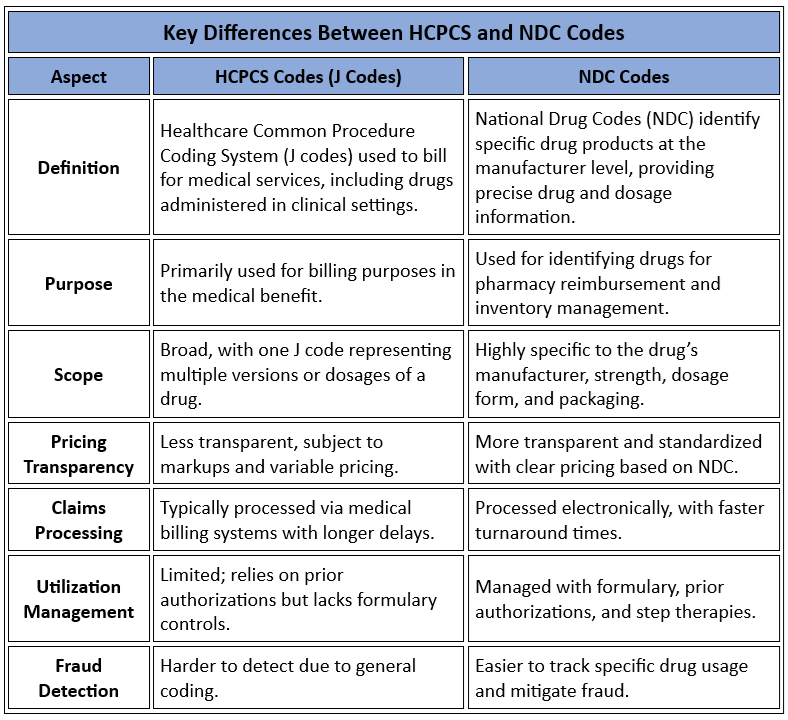
Specialty drug prices are notoriously high, and without proper oversight, costs can skyrocket. The first step in controlling spend is to ensure that you’re paying a fair price for the drugs your employees are using. Traditional PBMs may inflate costs through opaque pricing models, rebates, and spread pricing. To combat this, employers should:
- Demand transparency: Insist on a transparent, fiduciary model where your PBM charges only what the drug costs—nothing more, nothing less. This ensures there are no hidden markups or conflicts of interest.
- Implement cost-control measures: Require your PBM to actively negotiate with manufacturers for better pricing and rebates while using strategies like step therapy and prior authorization to ensure appropriate utilization. Additionally, price-lock guarantees and inflation protection clauses can offer predictability and protect against future cost hikes.
Clinical Appropriateness: Ensuring Optimal Use
Simply managing costs is not enough—it’s critical that specialty drugs are being used appropriately. This involves assessing whether the prescribed drug is the most clinically appropriate option for the patient based on their condition and overall health.
- Utilization management: Implement rigorous prior authorization protocols that evaluate the necessity of the specialty drug. This can prevent overprescribing or the use of more expensive drugs when equally effective, lower-cost options are available.
- Ongoing monitoring: Continuous evaluation of therapy effectiveness and adherence is key. Specialty drugs often require complex administration and management, so it’s important that patients are following their treatment plans and that the drugs are working as intended. Partner with a PBM that offers comprehensive patient support services and clinical oversight.
Drug Mix: Structuring an Efficient Specialty Formulary
The final piece of the puzzle is optimizing your specialty drug mix. This means ensuring that the drugs on your formulary provide the best value—both in terms of price and clinical outcomes.
- Formulary management: Work with your PBM to establish a tightly managed specialty drug formulary that prioritizes clinically effective, lower-cost alternatives such as biosimilars. Don’t just include drugs because they’re new—evaluate each drug for value and efficacy.
- Promote biosimilars: Biosimilars provide a great opportunity for cost savings. They are clinically equivalent to brand-name biologics but are often significantly less expensive. Ensuring they are included in your formulary and promoted as the preferred option can yield substantial savings.
Conclusion
By focusing on price transparency, ensuring clinical appropriateness, and optimizing your drug mix, self-insured employers can manage their specialty pharmacy benefits effectively. The goal is to strike the right balance between controlling costs and ensuring the best possible health outcomes for your employees. Working with a PBM that is aligned with your fiduciary responsibilities can help you achieve that balance and avoid the pitfalls of a profit-driven model.
For those seeking further guidance, TransparentRx offers a fiduciary model designed to reduce specialty drug costs while maintaining high standards of care. Reach out today to learn how we can help you create a sustainable and effective specialty pharmacy benefit.