One way the industry is responding is by diverting the national dialogue from price rollbacks to the notion of pay-for-value, which represents an area of common ground among healthcare policy stakeholders. The strategy aligns with healthcare payers’ focus on the total cost of care of their members and the pursuit of high-quality care — goals driven home by Medicare reforms that emphasize quality measurement and paying for value instead of volume.
The value discussion has intensified as drug development has shifted from primary care drugs to oncolytics and expensive specialty drugs, pressuring health plan pharmacy budgets. Health insurers and other healthcare purchasers are increasingly reluctant to pay for drugs that don’t work. That has led payers to experiment with outcomes-based contracts (OBCs) that hold pharmaceutical companies responsible for their products’ real-world performance.
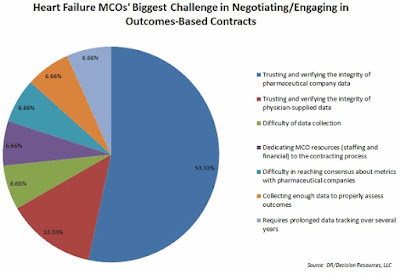 |
| [Click to Enlarge] |
Some of the most high-profile drugs to hit the market the past couple of years — including the cholesterol-lowering PCSK9 inhibitor Repatha and the hepatitis C drug Viekira Pak — have become the subject of OBCs. In February, Harvard Pilgrim Health Care, a Massachusetts-based health plan that had already inked a contract with Amgen on Repatha, announced another contract with Amgen for its rheumatoid arthritis drug Enbrel, which is facing impending competition from a biosimilar. The contract will tie the health plan’s payment for Enbrel to an effectiveness algorithm driven by six criteria, including patient compliance, switching or adding drugs, dose escalation, and steroid interventions.
The stars appear to be politically aligning to drive further growth of the pay-for-value trend. While the Trump administration has proclaimed a get-tough stance on drug pricing, it has also signaled its willingness to tackle the regulatory impediments that payers and drug companies say are holding back innovative contracting.
The commonly cited barriers — ranging from Medicaid best-price guarantees to anti-kickback rules to FDA regulations limiting the information manufacturers can share with payers — as well as the administrative difficulties of data sharing and collection, may be limiting the reach of risk-based contracting, but not stalling it.
More than one-third of the 40 managed care organizations (MCOs) surveyed by Decision Resources Group (DRG) indicated they were involved in OBCs, while another third said they expected to do such contracting in a year.