The 340B Drug Pricing Program, designed to provide discounted medications to healthcare organizations serving vulnerable populations, has long been a point of debate within the pharmaceutical industry. While its intent is noble, the ripple effects of 340B on commercial contracts are significant and complex, warranting a closer examination. Understanding the impact of the 340B program on commercial contracts is essential for navigating the complexities of the U.S. pharmacy reimbursement and distribution system.
The Basics of 340B
The 340B Program requires drug manufacturers to provide outpatient drugs at reduced prices to eligible healthcare organizations, known as covered entities. These entities include hospitals, community health centers, and clinics that serve many uninsured or low-income patients. The savings from these discounts are intended to support the covered entities’ ability to provide care to underserved communities.
Commercial Contracts and 340B: The Intersection
While the 340B Program is primarily aimed at supporting public health objectives, its impact on commercial contracts cannot be overlooked. Pharmaceutical manufacturers and commercial payers are increasingly scrutinizing the program’s influence on drug pricing dynamics and contract negotiations. This scrutiny stems from the fact that 340B pricing can distort the traditional market mechanisms that govern commercial drug pricing.
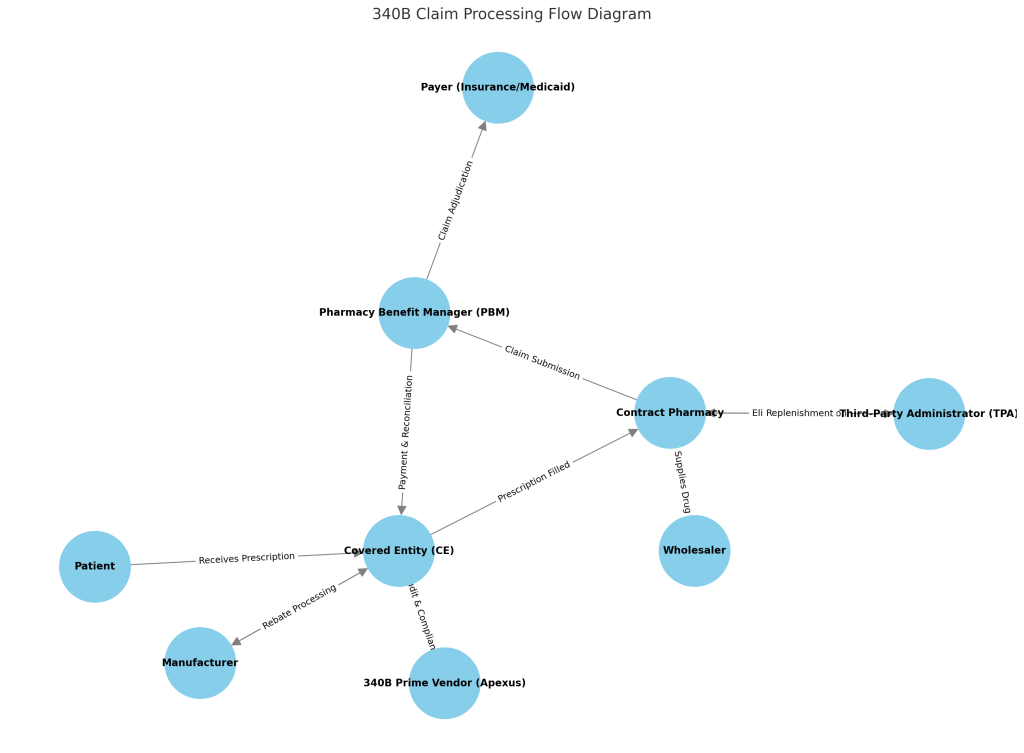
Key Impacts:
- Price Differentials and Contracting Challenges:
- One of the most significant impacts of 340B on commercial contracts is the creation of price differentials. Manufacturers are required to offer steep discounts under 340B, often leading to substantial differences between 340B prices and those negotiated in commercial contracts. This disparity can complicate negotiations between manufacturers and commercial payers, as the latter may seek to leverage 340B pricing in their contracts, potentially leading to lower reimbursement rates.
- Shift in Market Dynamics:
- The expansion of the 340B Program has also led to changes in market dynamics. Covered entities may purchase drugs at 340B prices but dispense them to insured patients, including those with commercial insurance. This practice, known as “spread pricing,” allows covered entities to benefit financially from the difference between the 340B acquisition cost and the higher reimbursement rates from commercial insurers. While this can provide financial relief to covered entities, it also introduces complexities in commercial contracting, as payers attempt to address the potential for inflated drug costs.
- Contractual Safeguards and Compliance:
- As the influence of 340B on commercial contracts grows, manufacturers and payers are increasingly incorporating safeguards and compliance measures into their agreements. These may include clauses that adjust reimbursement rates based on the covered entity’s 340B status or provisions that require transparency in how 340B savings are utilized. The goal is to ensure that the 340B Program’s benefits are aligned with its original intent without inadvertently undermining commercial contracting integrity.
Navigating the 340B Landscape
For stakeholders in the pharmaceutical industry, understanding the nuances of the 340B Program is crucial for effective contract negotiation and management. As the program continues to evolve, manufacturers and payers must remain vigilant in assessing its impact on pricing strategies and commercial relationships.
Best Practices:
- Transparency: Ensuring transparent communication between manufacturers, payers, and covered entities can help mitigate the potential for conflict and misaligned incentives in commercial contracts.
- Adaptive Contracting: Contracts should be flexible enough to adapt to the changing landscape of the 340B Program, including adjustments for price differentials and compliance requirements.
- Collaboration: Engaging in collaborative discussions with all stakeholders involved in the 340B ecosystem can lead to more equitable solutions that balance the program’s public health goals with the financial realities of the commercial market.
Conclusion
The 340B Drug Pricing Program, while a critical tool for supporting healthcare organizations serving vulnerable populations, has far-reaching implications for commercial contracts in the pharmaceutical industry. As the program continues to shape the market, stakeholders must navigate the challenges and opportunities it presents with a strategic and informed approach.