FDA Biosimilar Plan Offers Employers Saving Strategy Solution
 |
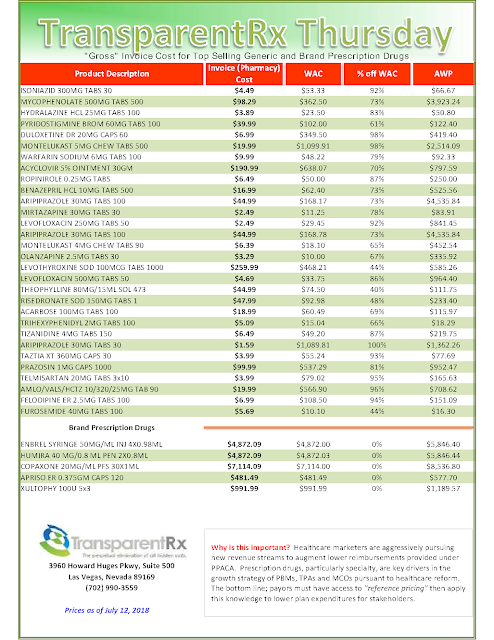
| List of Biosimilar Drugs and their Marketing Status (Updated September 14, 2017) |
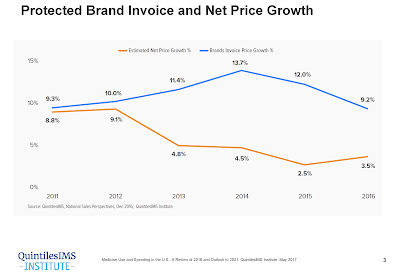
In 2017, biosimilars generated $3 billion worldwide in revenue and present growing market competition to the specialty biotech market. Escalating specialty drug costs present a challenge for many employers who struggle to balance health care spend with business financial livelihood.
Based on 2017 FDA research, the delayed market launch of nine biosimilars represented $4.5 billion in potential savings. As part of the FDA Biosimilar Action Plan, biosimilar drugs are poised to offer a solution that could help deliver significant cost advantages without compromising therapeutic efficacy, safety, or quality.
Biosimilars are biologic drugs that are highly similar in structure and function to existing FDA approved reference drugs. With fully established FDA safety and efficacy data, reference drugs are used as the benchmark to which biosimilars are compared to.
For example, Neupogen is the reference drug for both biosimilar products Zarxio and Granix. Biosimilars must demonstrate no clinically meaningful efficacy differences and equal safety to gain FDA approval. Biosimilars should not be viewed as generic drugs, but rather an alternative form of brand medications that would usually be categorized as specialty.