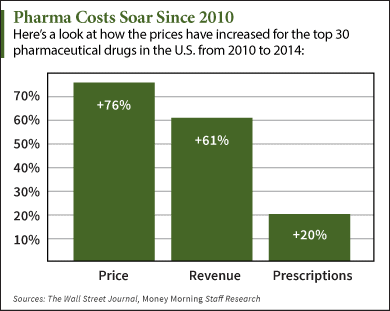
 |
| [Click to Enlarge] |
A brand name medication, also known as “pioneer” or “branded” medication, is made by one manufacturer (single source) under a patent issued by the United States Patent Office. The manufacturer of the branded medication has a 20-year exclusive patent during which no other manufacturer is allowed to produce the exact same product. Examples of brand name medications include Lipitor, Exubera, and Humira.
A generic medication, or multi-source medication, is a medication produced by multiple manufacturers. Generic products are allowed to be marketed after the brand-name medication loses patent protection. Generic manufacturers vie to be the first one on the market, as they are given a six-month exclusivity period to regain development costs.
After six months, any manufacturer may apply for a license to market a generic version of the pioneer product. Pricing discounts, which are typically 10 to 15 percent in the first six months, drop to 50 to 70 percent or more after the six-month exclusivity period.
Multi-source simply means that multiple manufacturers produce the same medication. Some payers refer to a multisource medication as the first generic on the market after the pioneering brand name medication loses patent protection. Within the industry, the terms generic medication and multi-source medication are used interchangeably.
Authorized generics are considered brand drugs under a generic label. Put simply, a brand drug manufacturer supplies its drug to a generic firm and allows the firm to market the product under a different label for royalties. Brand companies also can create their own companies or subsidiaries to manufacture these authorized generics.
By taking either route, these authorized generics can compete with the first generic drug maker during their 180-day exclusivity period. The implications of such actions create a price war that reduces the price of both generics in the 180-day period, thereby reducing the market share and profitability for the generic manufacturer.
The pharmaceutical industry may not face government price regulations, but it is subject to heavy safety regulations, which makes it difficult for competitors to enter the market. Because of regulations that are ofttimes correctly established for safety and efficacy, it results in extremely high barriers to entry. It could take four or five years for a generic manufacturer to develop a competing product. A lack of competition inevitably leads to higher prices in a capitalist society.
In summary, there are at least five scenarios which lead to generic medications costing nearly as much as their brand name equivalents:
1. Rent-Seeking
2. Lack of Competition
3. High Barriers to Entry
4. Limited Distribution
5. Intellectual Rights
In order to minimize the sting from rising drug costs, payers must do two things. First, require a fiduciary standard from their pharmacy benefit managers. It it the highest standard of care and leaves little open for interpretation or arbitrage. Second, implement hyper-aggressive strategies to control drug costs. These include but are not limited to: utilization management, step therapy, quantity limits, cost sharing, and carve-out.
