“Don’t Miss” Webinar: How to Slash PBM Service Costs, up to 50%, Without Changing Vendors or Benefit Levels
Here is what some participants have said about the webinar.
“…Great presentation! I had our two partners on the presentation as well. Very informative.” Nolan Waterfall, Agent/Benefits Specialist
A snapshot of what you will learn during this 30 minute webinar:
-
- Hidden cash flows in the PBM Industry such as formulary steering, rebate masking and differential pricing
- How to calculate cost of pharmacy benefit manager services or CPBMS
- Specialty pharmacy cost-containment strategies
- The financial impact of actual acquisition cost (AAC) vs. effective acquisition cost (EAC)
 |
| Recertification Credit Hours: 2 |
- Why mail-order and preferred pharmacy networks may not be the great deal you were sold
TransparentRx
Tyrone D. Squires, MBA
3960 Howard Hughes Pkwy., Suite 500
Las Vegas, NV 89169
866-499-1940 Ext. 201
P.S. Yes, it’s recorded. I know you’re busy … so register now and we’ll send you the link to the session recording as soon as it’s ready.
Reference Pricing: “Gross” Invoice Cost for Popular Generic and Brand Prescription Drugs (Volume 187)
The costs shared here are what the pharmacy actually pays; not AWP, MAC or WAC. The bottom line; payers must have access to actual acquisition costs or AAC. Apply this knowledge to hold PBMs accountable and lower plan expenditures for stakeholders.
How to Determine if Your Company [or Client] is Overpaying
Step #3: Compare approximately 10 to 20 prescription claims against the price list to confirm contract agreement. It’s impractical to verify all claims, but 10 is a sample size large enough to extract some good assumptions.
Step #4: Now take it one step further. Check what your organization has paid, for prescription drugs, against our acquisition costs then determine if a problem exists. When there is a 5% or more price differential (paid versus actual cost) we consider this a problem.
Multiple price differential discoveries means that your organization or client is likely overpaying. REPEAT these steps once per month.
— Tip —
When better pricing is discovered the contract language should stipulate the client be indemnified. Do not allow the PBM to limit the market check language to a similar size client, benefit design and/or drug utilization. In this case, the market check language is effectually meaningless.
Hospital Impact—Pharmacy benefit managers are a lot like car dealers
 |
| Click here to learn more |
The same system applies to buying lifesaving prescriptions. Drug manufacturers set a list price, but consumers rarely pay that, and how much drugs should really cost is uncertain. That’s because of pharmacy benefit managers (PBMs), large corporations wielding enormous power over which drugs you get. Just three big PBMs control nearly 80% of the market, using their size and influence to negotiate drug prices with manufacturers. However, big PBMs have mixed incentives around containing drug costs and managing premium costs, while maximizing profits from their own in-house pharmacies.
Tyrone’s comment: While I agree with the premise of this article’s title, there is one small yet very important distinction that must be made. That distinction is “non-fiduciary” pharmacy benefit managers are a lot like car dealers. Moreover, how do we classify those trusted advisors who steer their clients to these car dealers or non-fiduciary pharmacy benefit managers without first having won radical transparency? PBMs are not created equally with some volunteering radical transparency and others much less so. Be sure to do your [plan sponsors] homework which requires going far beyond simple spreadsheet analysis and 25 page RFPs. Who is watching the watchers?
In the past few years, PBMs have ramped up their mail-order and specialty pharmacies to lock down profits and exclude independent pharmacies. Increasingly, they’re exploiting a Medicare provision allowing them to charge “direct and indirect remuneration fees” to pharmacies within their network. They’re ratcheting up the use of these fees—ostensibly part of Medicare reimbursement—to price out competition: local independent pharmacies, where pharmacists have personal relationships with patients and partner with referring providers to support patient care.
Impact of Biosimilar Approvals on the Pharmaceutical Market
Although biologics account for less than 1% of all dispensed prescription drugs in the United States, it accounts for one-fourth of all prescription spending. Concern surrounding high price tags of biologics continues to rise, and biosimilars offer the potential to provide patients with less costly alternatives.
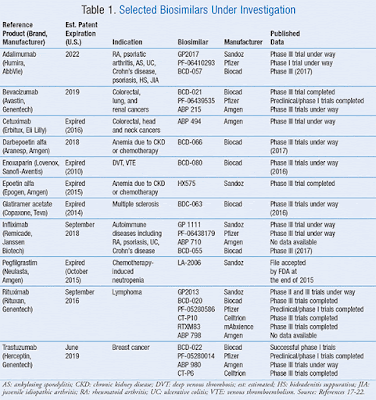 |
| Table1: some of the trials being conducted on biosimilars |
In an interview with Specialty Pharmacy Times, Yogesh Soneji, a partner at Trinity Partners, discusses the future of pharmacy and the effect of biosimilar approvals.
SPT: What impact can biosimilar approvals have on the pharmaceutical marketplace?
Soneji: I think these are exciting times. It’s almost a new era for the industry in general, [with] the launch of recent biosimilars and some uncertainty around what happens in the near term. TABLE 1 lists some of the trials being conducted on biosimilars, and if successfully completed, these products may become available in the near future.
When you think about the marketplace there are so many different players. A way I think about this is there are going to be 2 camps: the brand to whom a biosimilar is being launched against and then the biosimilars themselves. The impact is going to be different depending on what side of the fence you are on.
For the branded companies that have biologics today, it’s going to be a little more uncertain in the near term of what happens, because besides the upcoming launch timing of biosimilars, they also have to have to guess what the pricing (of biosimilars) is going to be, what the biosimilars will discount their drugs at, and there is going to be some uncertainty, at least in the near term, on how to mitigate that. On the other hand, for the companies that are actually launching these biosimilars, that represents a whole world of opportunities.
SPT: How will biosimilars affect future drug prices?
Soneji: Only time will tell, but if you even see what is going on recently, you have biologics with different price points, with one in the 15% discount list price and another over 30% discounted list price. I think it’s already been mentioned that the branded players, like Remicade, have been more aggressive on contracting on their own drug and getting better contracting in order to safeguard their business in the near term. I think there is no doubt that we will see prices go down and spiral, but the question isn’t about whether they will or not. I think the question is how fast and what that path looks like. If it’s going to happen very quickly or if it’s going to happen in a steadier manner over time.
I do think in the long run, biosimilars will drive down prices. One key aspect with pricing that’s a little more complicated in the drug industry, as you might have seen, is it is not very transparent about what the price of the drug is.
We see list prices mentioned regularly in press releases about Inflectra and what their list price is compared to the list price of Remicade. But I think the key here is for us to keep an eye out for not just the list price, but what is the net price, which takes into account the rebates and discounts which can be negotiated on a payer basis, and what happens to those and what path those take. Because that is what drives decisions, especially from the payer side. Which drugs they put on formulary, which drugs they prefer to use at the end of the day, what really matters with pricing is net price that payers have to pay including contracts/rebates and discounts.
Reference Pricing: “Gross” Invoice Cost for Popular Generic and Brand Prescription Drugs (Volume 186)
The costs shared here are what the pharmacy actually pays; not AWP, MAC or WAC. The bottom line; payers must have access to actual acquisition costs or AAC. Apply this knowledge to hold PBMs accountable and lower plan expenditures for stakeholders.
Step #3: Compare approximately 10 to 20 prescription claims against the price list to confirm contract agreement. It’s impractical to verify all claims, but 10 is a sample size large enough to extract some good assumptions.
Step #4: Now take it one step further. Check what your organization has paid, for prescription drugs, against our acquisition costs then determine if a problem exists. When there is a 5% or more price differential (paid versus actual cost) we consider this a problem.
Multiple price differential discoveries means that your organization or client is likely overpaying. REPEAT these steps once per month.
— Tip —
When better pricing is discovered the contract language should stipulate the client be indemnified. Do not allow the PBM to limit the market check language to a similar size client, benefit design and/or drug utilization. In this case, the market check language is effectually meaningless.
Cost Containment Strategies and Vendor Contracting Employers’ Top Health Plan Concerns
 |
| Click to learn more |
When asked to rank cost-management strategies implemented by group health plans in 2017, survey respondents to a recent survey by the Segal Group listed prescription drug cost management strategies and improved vendor contracting as plan sponsors’ main concerns. “Using specialty pharmacy management; intensifying pharmacy management programs; contracting with value-based providers; increasing financial incentives in wellness design; and adopting a high deductible health plan (HDHP),” were the top five recorded by plan sponsors.
The findings, according to the study, demonstrate how sponsors are pushing utilization by encouraging high quality, low cost providers, as well as following strategies to lower costs, including the “use of custom and limited provider networks, expansion of dedicated primary care clinics that are on or near work-sites and, for some industries, continued migration to tax advantaged health savings accounts (HSAs) and HDHPS.”
Tyrone’s comment: Brokers, consultants and plan sponsors are better served when they roll up their sleeves and become more knowledgeable about the inner-workings of PBM services. Unfortunately, most purchasers are essentially winging it picking up secondhand information as they go hoping something sticks. A 250 question RFP doesn’t guarantee much if you can’t get important disclosure details to hold up in the contract language, for example. Education is the key to eliminating overpayments for pharmacy services. Remember the fight is not about you or me, it’s about the patients who rely on us to make affordable and quality healthcare available to them – this very important point often gets lost during the procurement process. Success dictates you acquire knowledge far beyond the functional role of a PBM.
In response to these results, Edward Kaplan, national health practice leader for Segal, suggests sponsors adopt a “three-pronged approach to the challenge of health care cost management that encompasses vendor management, plan design management and population health management.”
Pfizer Files Lawsuit Against J&J, Claiming Anticompetitive Practices
 |
| Source: Pink Sheet Pharma Intelligence |
Pfizer filed suit against Johnson & Johnson (J&J) on Wednesday for allegedly using anticompetitive practices to prevent less expensive versions of its rheumatoid arthritis (RA) drug to thrive in the budding biosimilar market.
J&J has sold the injectable biologic Remicade for nearly 2 decades, generating $4.8 billion in US sales last year alone. This injectable biologic drug is widely used to treat RA, Crohn’s disease, and other inflammatory disorders, according to the Chicago Tribune.
Pfizer’s Inflectra was the second biosimilar to receive FDA approval.
If Pfizer wins the case against J&J, it could pave a new path that would discourage brand name companies from cutting deals with insurers to limit competition in the biosimilar marketplace, according to the Chicago Tribune. However, if J&J comes out on top it could continue to fuel strategies that thwart competition, according to the article.
Tyrone’s comment: While this is a first in the biologics industry, these types of lawsuits (generic vs brand manufacturers) are not uncommon. It’s a clear indication biosimilars are a serious threat to brand competitors and will soon come to market in mass. J&J knows the future of biotech includes biosimilars so they are delaying the inevitable in an attempt to hold on to the cash cow for as long as possible. Because cost will eventually come down as a result of these lawsuits and innovation, this is a good thing for self-funded plan sponsors – stay tuned.
“This is the first lawsuit that is challenging anti-competitive behavior in the biologics industry, and that is very important because this is the wave of the future––this is where a lot of the innovation is taking place today,” Michael Carrier, law professor at Rutgers Law School, told the Chicago Tribune. “It really is an uncharted path, in terms of what competition should look like going forward.”
Reference Pricing: “Gross” Invoice Cost for Popular Generic and Brand Prescription Drugs (Volume 185)
The costs shared here are what the pharmacy actually pays; not AWP, MAC or WAC. The bottom line; payers must have access to actual acquisition costs or AAC. Apply this knowledge to hold PBMs accountable and lower plan expenditures for stakeholders.
Step #3: Compare approximately 10 to 20 prescription claims against the price list to confirm contract agreement. It’s impractical to verify all claims, but 10 is a sample size large enough to extract some good assumptions.
Step #4: Now take it one step further. Check what your organization has paid, for prescription drugs, against our acquisition costs then determine if a problem exists. When there is a 5% or more price differential (paid versus actual cost) we consider this a problem.
Multiple price differential discoveries means that your organization or client is likely overpaying. REPEAT these steps once per month.
— Tip —
When better pricing is discovered the contract language should stipulate the client be indemnified. Do not allow the PBM to limit the market check language to a similar size client, benefit design and/or drug utilization. In this case, the market check language is effectually meaningless.
New Hep C Pricing Model: A Game Changer for Self-Funded Employers
 |
| Click to Learn More |
For as long as I can remember pharmacy benefit managers have blamed pharmaceutical manufacturers for the high cost of prescription drugs. Drugmakers do share some of the responsibility but so to do pharmacy benefit managers.
Because it essentially eliminates the PBM mark-up, AbbVie’s pricing strategy for its new Hep C drug Mavyret is disruptive to the traditional PBM revenue model. Abbvie is not going to pay a rebate and instead price it [rebate] back into the list price in the form of a significant list price discount. This move forces non-fiduciary PBMs to consider Mavyret for inclusion in their formularies even though the pricing strategy is not aligned with their own interest of needing manufacturer revenue to protect top line revenue.
The looming question is will non-fiduciary PBMs include Mavyret as a preferred drug in the HCV therapeutic class on their formularies. It is less costly and as efficacious as any other drug in the HCV class so it should be a no brainer right? Wrong. If the PBM decides to exclude or list the drug as non-preferred it’s likely because their interests aren’t aligned with those of their clients’. Let’s wait and see.




