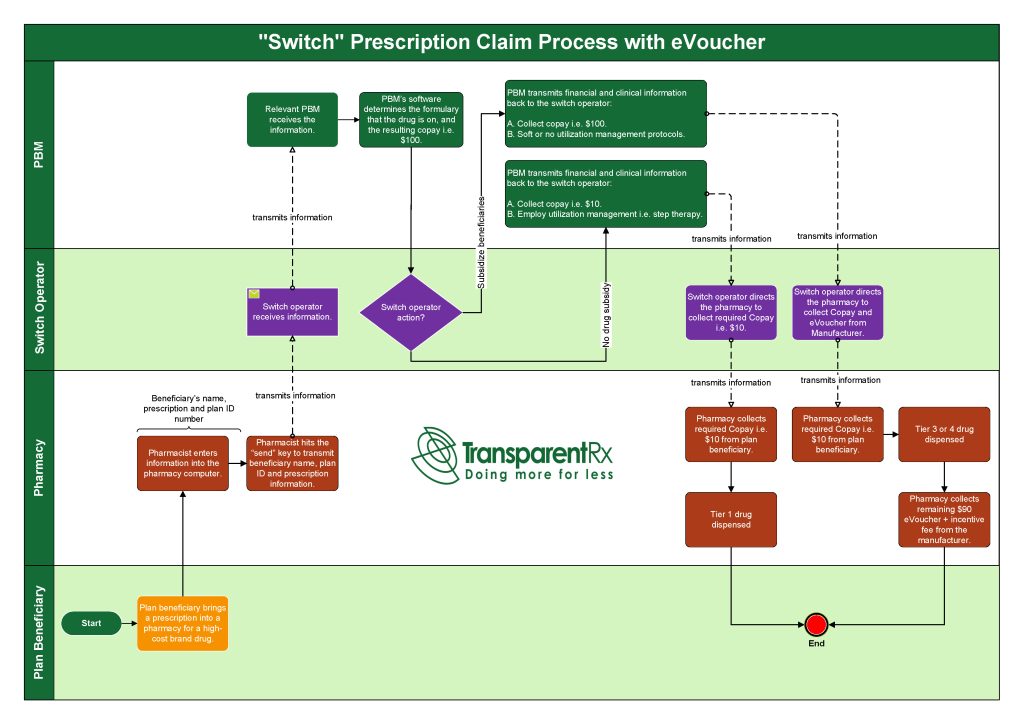
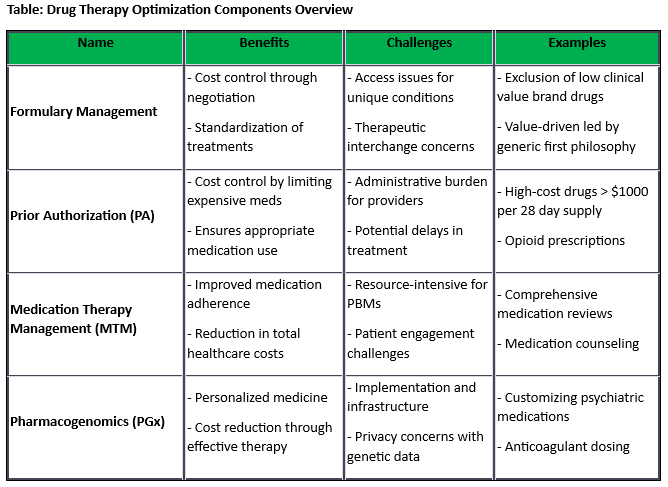
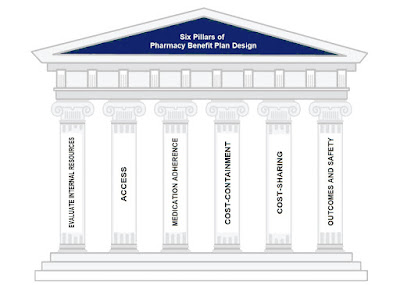
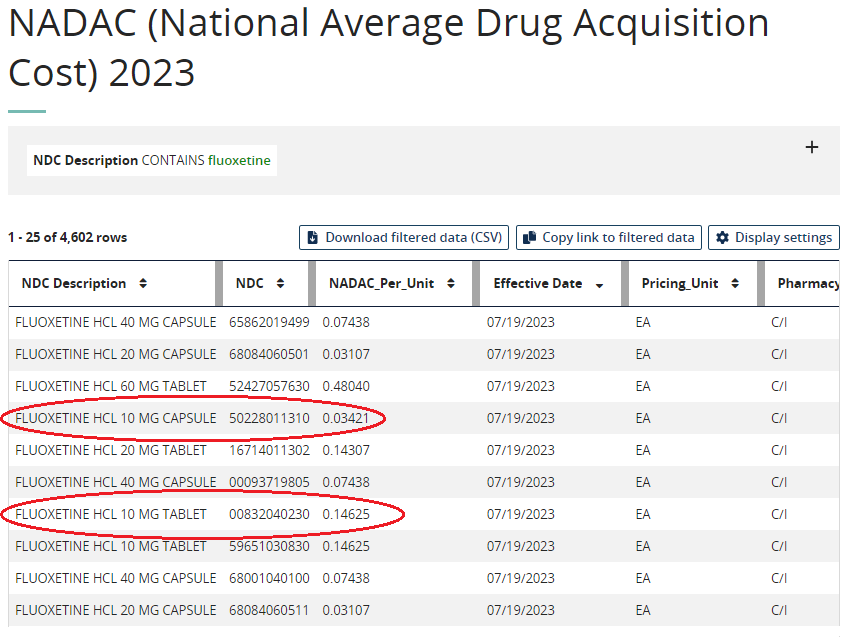
ICER Updates Assessment Framework to Include New Measures of Value [Weekly Roundup]
ICER Updates Assessment Framework to Include New Measures of Value and other notes from around the interweb:
- ICER Updates Assessment Framework to Include New Measures of Value. The Institute for Clinical and Economic Review (ICER) has updated the framework it uses to assess the cost-effectiveness and value of prescription drugs. The organization has for 15 years provided information that has assessed the available evidence about a product’s value. Its first formal value assessment framework was published in 2015 with updates in 2017 and 2020. One of the more significant changes will be to include an assessment of a drug’s impact from a societal perspective, for example on patient and caregiver productivity, what ICER calls “non-zero” inputs. Clinical trials often do not gather this information and health economists have been conservative when there is no data, which leads to a “zero” input on measures of societal impact.
- Specialty drug cost trend projected to be nearly 15 percent. Specialty drug trend alone is projected to be nearly 15 percent, driven by higher utilization of new high-cost specialty drugs replacing lower-cost therapies. Diabetes, autoimmune disease, and psoriasis have been the top three disease indications for prescription drugs over the past few years. However, since the first quarter of 2021, anti-obesity medications have shown the greatest growth, climbing 114 percent. This is due to many factors, including the off-label, weight-loss use stemming from social media buzz and exponential market investments in anti-obesity drugs as well as the American Diabetes Association recommending GLP-1 medication to reduce health complications. GLP-1 drugs will account for more than half of all diabetes drug therapies claim costs by the end of this year.
- Competition in Commercial PBM Markets and Vertical Integration of Health Insurers with PBMs: 2023 Update. Based on 2020 data and newly acquired 2021 data for people with a commercial drug benefit tied to a medical benefit and the PBMs used by insurers, the updated analysis presents market insight on five PBM services performed for insurers: rebate negotiation, retail network management, claim adjudication, formulary management, and benefit design. Insurers face a make-or-buy decision—they can perform these functions in-house or buy them from a PBM. The AMA Policy Research Perspectives report, “Competition in Commercial PBM Markets and Vertical Integration of Health Insurers with PBMs: 2023 Update”, found that insurers largely use a PBM for three of them—rebate negotiation, retail network management and claims adjudication—and therefore assessed market competition for those three product markets.
- STAT News investigation takes deep dive into PBM broker conflicts of interest. Employers across the country — from big names like Boeing and UPS to local school systems — pay consulting firms to handle a straightforward task with their prescription drug coverage: Get the best deals possible, and make sure the industry’s middlemen, known as pharmacy benefit managers, aren’t ripping them off with unfair contracts. But a largely hidden flow of money between major consulting conglomerates and PBMs compromises that relationship, a STAT investigation shows. Some consulting firms often are getting paid more — a lot more — by the PBMs and health insurance carriers that they are supposed to scrutinize than by companies they are supposed to be looking out for.